Cannabinoids in the Context of the Russian-Ukrainian War: Could Cannabis Improve the Mental State of Ukrainians in the Face of a Full-Blown Invasion?
-
14 August 2025Переглянуто: 3507
Intro
In the context of the ongoing war and growing mental health challenges in Ukraine, the issue of finding new ways to support people who have experienced traumatic events among veterans and those who did not participate in hostilities is particularly acute. The evidence base of preclinical and initial clinical studies shows the significant therapeutic potential of cannabinoids in reducing the symptoms of PTSD, anxiety, and sleep disorders, which are the problems that Ukrainians are facing on a large scale during the full-scale Russian aggression. So can cannabis, at least in part, become a tool for preserving the mental health of people affected by the war? In this article, I will try to give the most comprehensive answer to this painful, yet relevant question.
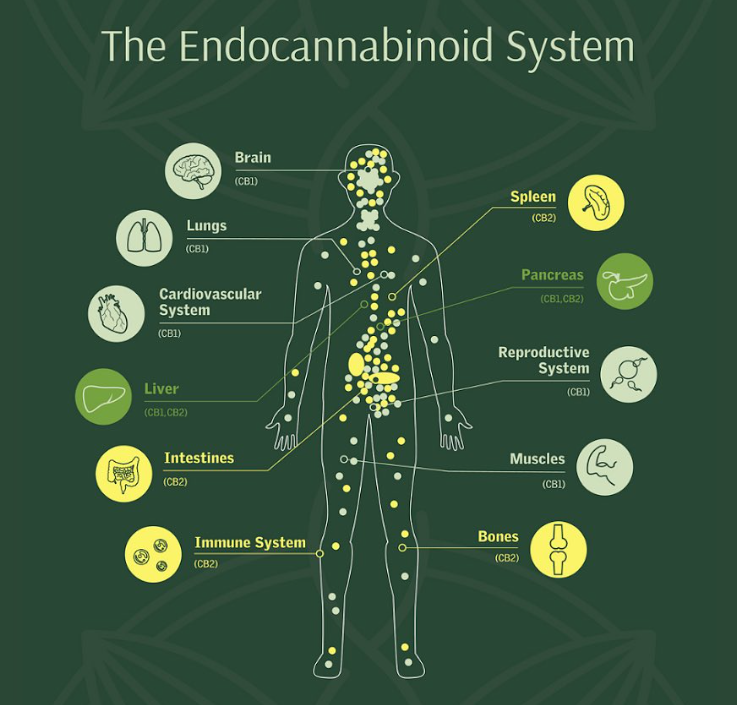
What is the endocannabinoid system?
Further studies indicate that ECS is not limited to short-term changes - it is also involved in longer-term forms of synaptic plasticity, such as long-term potentiation (LTP) and depression (LTD), in many brain regions, including the hippocampus, amygdala, and prefrontal cortex. These changes underlie learning, emotional regulation, and memory (Maldonado et al., 2006; Di Marzo, 2009; Sidhpura & Parsons, 2011; Bitencourt & Takahashi, 2018).
Thus, the endocannabinoid system appears to be a powerful module for regulating brain neurophysiology, from the stages of its development to the finest settings of emotional experience and memory.
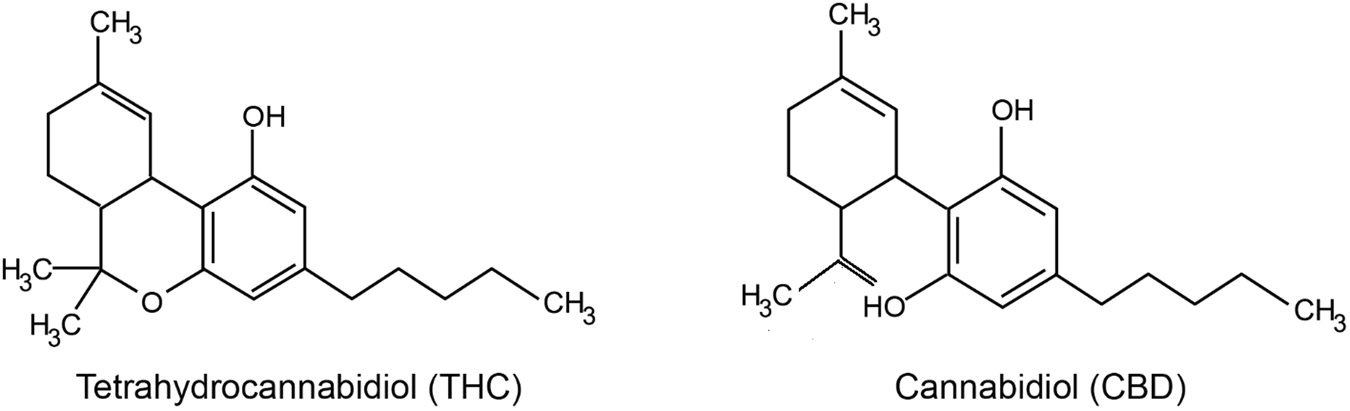
Exogenous phytocannabinoids: CBD and THC
Cannabis contains several biologically active compounds, among which the most famous are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD).THC — is the main psychoactive component of cannabis that causes a state of euphoria or "high", At the same time, we see that it has analgesic, anti-inflammatory, anticonvulsant, antiemetic properties, as well as the ability to stimulate appetite (Chayasirisobhon, 2020; Harbich, Michalak, & Michalak, 2024). Its main mechanism of action is the activation of CB1 receptors - the main cannabinoid receptors in the central nervous system (Wright, Di Ciano, & Brands, 2020).Modern forms of use
Although inhaled and smoked forms of cannabis are often used by patients, they are not standardized as pharmaceutical products due to significant dose variations, complex regulatory restrictions, and safety concerns(Stella et al., 2021).
The only FDA-approved plant cannabinoid is Epidiolex — an oral CBD solution designed to treat epileptic syndromes(Stella et al., 2021). Synthetic analogs of THC - dronabinol and nabilone - are available in the form of oral capsules(Stella et al., 2021).
For the convenience of patients and to avoid significant liver metabolism, oropharyngeal CBD tablets are being developed that disperse rapidly in the oral cavity. Furthermore, transdermal gels, dermal sprays, and nanospheric systems are in clinical trials, providing controlled release of cannabinoids without oral or inhalation routes of administration.(Stella et al., 2021).
Traditional topical (liniments) and galenic forms - gels, oils, creams, ointments - often contain CBD, THC or other cannabinoids (e.g. CBG) in combination with excipients that improve skin permeability and stability of the product(Kaczorová et al., 2023).


Cannabinoids and anxiety
Anxiety disorders are the most common mental illnesses in the world, creating a significant social cost and economic burden. They are accompanied by panic attacks, avoidant behaviors, and reduced overall well-being, leading to poorer interpersonal relationships, higher unemployment, and increased risk of suicide.(Wright, Di Ciano, & Brands, 2020).
The first clinical trials back in the 1980s showed that CBD can reduce the anxiogenic and psychoactive effects of THC in healthy volunteers (Wright, Di Ciano, & Brands, 2020).
Neuroimaging studies of acute CBD administration have found modifications in cerebral blood flow in key structures associated with anxiety: the amygdala, hypothalamus, hippocampus, and cingulate cortex (Wright, Di Ciano, & Brands, 2020).
Thus, from preclinical models to the first clinical and neuroimaging data, the accumulating evidence clearly points to the multifaceted anxiolytic potential of CBD, which is dose-dependent, context-dependent, and targeted neuronal networks.
Cannabinoids and PTSD
Post-traumatic stress disorder (PTSD) — is a chronic condition that can develop after experiencing a traumatic event and is manifested by sleep disturbances, cognitive changes (e.g., intrusive recall of the event), mood disturbances, emotional instability, and reduced social skills(Bitencourt & Takahashi, 2018). Currently, anxiolytics and antidepressants are used to treat PTSD, but their effectiveness is limited and their side effects are significant(Berger et al., 2009; Shin et al., 2014; Bernardy & Friedman, 2015; Bitencourt & Takahashi, 2018).Limitations faced by scientists and doctors
While CBD is generally considered well tolerated, a recent meta-analysis of 1-14 week studies showed that the most common adverse effects (AEs) were sedation, decreased appetite, diarrhea, and drowsiness, with more severe AEs more commonly observed at high doses and in patients with epilepsy - a possible contribution to this being drug-drug interactions with antiepileptic drugs(Chesney et al., 2020; Gaston et al., 2017; Geffrey et al., 2015; Morrison et al., 2019). In studies of other indications, where CBD was used in healthy volunteers, difference from placebo was found only in terms of diarrhea(Chesney et al., 2020).The issue of serotonin mechanisms remains controversial: Rock et al. (2012) did not confirm a direct agonistic effect of CBD on 5-HT1A, suggesting allosteric or intracellular modifications; there is no experimental confirmation of these mechanisms in living organisms (Ibeas Bih et al., 2015).
Therefore, further research should not only expand the clinical range of CBD doses and administration regimens, but also elucidate its molecular targets and accompanying effects to fully assess its therapeutic potential in various neuropsychiatric conditions.
Mental challenges during the war: stress, anxiety, depression
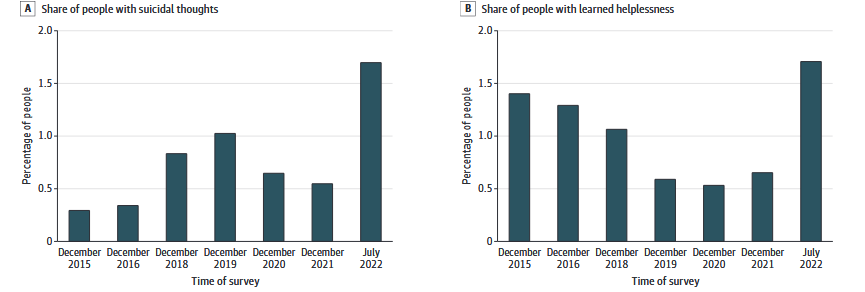
Stress — is a normal body reaction to external threats, but in the context of a full-scale invasion, its level in the population of Ukraine averaged 7.55 out of 16 possible points six months after the start of hostilities (Kurapov et al., 2023).Experience of other countries
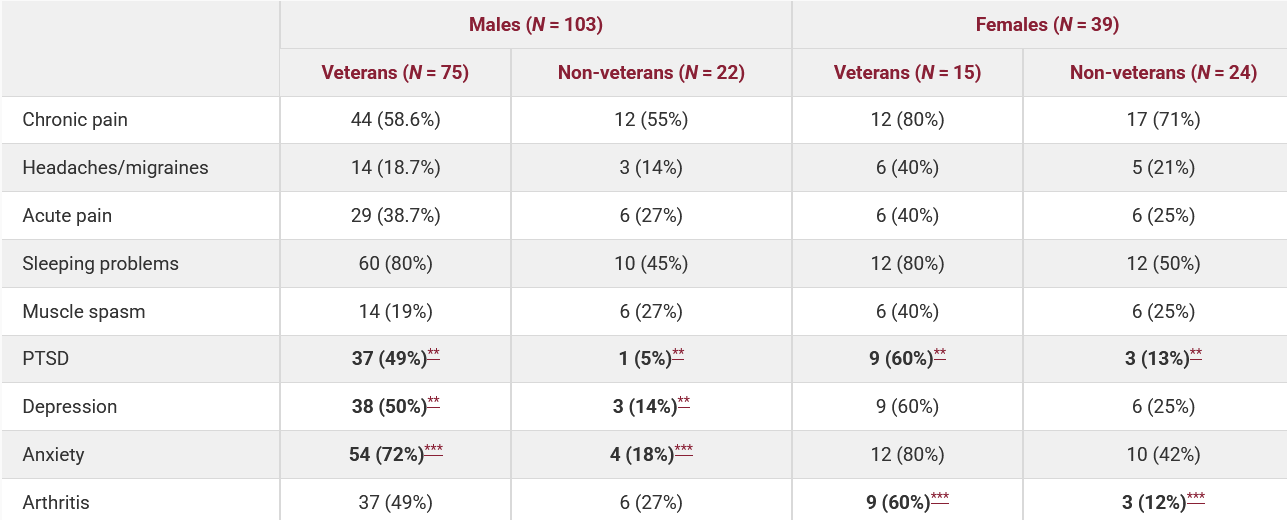
In the United States, the use of cannabis for the treatment of PTSD among veterans has been studied most thoroughly. It was found that approximately 44% of veterans with mild traumatic brain injuries use cannabis on a monthly basis, five times more than the general population (8.6%) (Utter et al., 2023). Among those who seek help, the percentage of cannabis users for medical or recreational purposes reaches 40-45% (Betthauser et al., 2015; Metrik, J. et al, 2018). Although the first placebo-controlled study conducted by MAPS did not show any benefit of smoking cannabis over placebo (Bonn-Miller et al., 2021), more recent data show that veterans who used prescription cannabis were 2.5 times more likely to no longer meet the criteria for a PTSD diagnosis (VFW, 2021). At the same time, VA policy prohibits the prescription of medical cannabis due to its federal Schedule I classification, and veterans are forced to seek it out on their own in state programs or on the illegal market (Politico, 2021). It was only in late 2024 that the FDA authorized a new placebo-controlled study, MJP2, involving 320 veterans to study cannabis with high THC concentrations in real-world settings (Stars and Stripes, 2024; The Psychiatrist, 2024).

At the same time, the problem of access to cannabis remains open for Ukrainian citizens affected by the war. Medical cannabis was legalized in August 2024, but PTSD was excluded from the final list of diseases, although the Ministry of Health estimates the potential target audience at 6 million people (Business Insider, 2023; Le Monde, 2024; Cannabis Health News, 2024). The Cannabis with Ukraine campaign seeks to distribute therapies to the wounded and children of the dead, but there are no clear mechanisms to ensure access for civilians (Soft Secrets, 2025).
Finally, while countries demonstrate different levels of political support and scientific basis, the overall challenge remains balancing accessibility, evidence of efficacy, and long-term safety of cannabinoid therapies for veterans and civilians affected by combat.
Legal and medical status of cannabis in Ukraine
On February 16, 2024, President of Ukraine Volodymyr Zelenskyy signed the Law of Ukraine No. 3528-IX "On Amendments to Certain Laws of Ukraine on State Regulation of the Turnover of Plants of the Cannabis Genus". The law came into force on August 16, 2024, marking a historic moment for medical practice in Ukraine (Cabinet of Ministers of Ukraine, 2023; Suspilne, 2025; Ukrinform, 2024).
Draft law No. 7457, as it was known at the stage of consideration, was adopted by the Verkhovna Rada on December 21, 2023, in the second reading and in general, with the support of 248 deputies. The document regulates the circulation of cannabis exclusively for medical, industrial, scientific, and scientific and technical purposes, leaving recreational use under the ban (Rada, 2025; BBC Ukraine, 2023).
The Ministry of Health of Ukraine has approved the list of diseases and conditions for which medical cannabis will be prescribed by Order No. 1586 of September 13, 2024. The list is based on the most up-to-date research and evidence of efficacy and will be reviewed periodically (МЗ, 2024; ТСН, 2024).
The approved list includes:
Chronic or neuropathic pain and/or spasticity caused by:- Malignant neoplasms(C00-C97)
- Diabetic neuropathy(E10.4-E14.4)
- Multiple sclerosis (G35)
- Lesions of the trigeminal and facial nerves (G50, G51)
- Neuralgia due to shingles (G53.0)
- Spinal cord injuries and intracranial injuries
- Cerebral palsy and other paralytic syndromes
Other indications include:
- Nausea and vomiting due to chemotherapy
- Parkinson's disease and de la Tourette syndrome
- Refractory epilepsy
- Pediatric convulsive syndromes (Lennox-Gastaut, Dravet, tuberous sclerosis)
- Weight loss in HIV infection
- Other diseases with the conclusion of the medical advisory commission (MOH, 2024; MedPlatforma, 2024).
Medical cannabis-based medicines can be obtained exclusively with an electronic prescription from a primary or specialized healthcare provider. Data on prescriptions are entered into the electronic healthcare system (EHR), which ensures full transparency of the process (MoH, 2024; Hromadske Radio, 2025).
There are special restrictions for children: the ratio of cannabidiol to tetrahydrocannabinol should be more than 20:1, and the maximum daily dose should not exceed 25 mg/kg body weight. The drug is not prescribed to pregnant women, women while breastfeeding, or patients with a tendency to psychotic disorders (The Village, 2025).
In January 2025, the first medicines based on medical cannabis were officially registered in Ukraine - oral drops from the Spanish brand Kuralif. This was an important milestone after many years of struggle by patient organizations for access to treatment (Gvara Media, 2025; LB.ua, 2025).
Stigmatization and social and legal barriers
The biggest problem is people's misunderstanding and ignorance of the differences between medical and recreational cannabis. According to representatives of patient organizations, there is a persistent stigma in society when the concepts of medical and recreational cannabis are deliberately mixed, which creates misunderstandings about the safety and effectiveness of medicines (Ukr.radio, 2024).
A 2020 study among Ukrainian pharmacy students found that almost half of respondents were not sufficiently informed about the therapeutic properties of cannabis, although more than 90% believed that materials on the medical properties of cannabis should be included in the curriculum (Kovalenko et al., 2020).
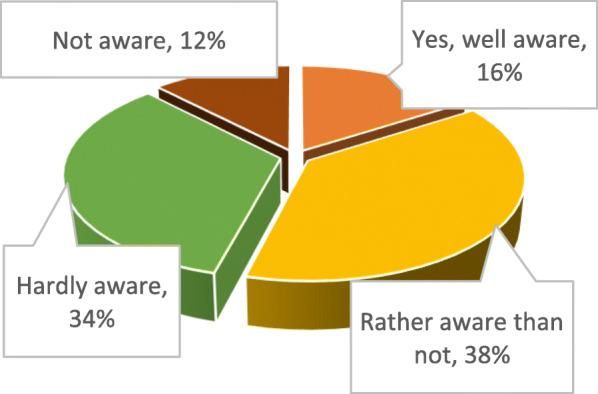
Kovalenko, A., Pakhomov, I., Horoshko, O., Kalinichenko, V., & Bezditko, P. (2020). Perspectives on formation of medical cannabis market in Ukraine based on holistic approach. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC7819340/
Patient organizations insist on including PTSD in the official list of indications. Currently, this condition can be treated with medical cannabis only with the conclusion of a medical advisory committee in hospitals with a scientific base, which limits access for many veterans(LB.ua, 2025).
The lack of proper training for medical professionals creates additional obstacles. Charitable organizations, together with the NHSU Academy, are preparing online training for Ukrainian doctors so that they can adopt modern treatment regimens from their foreign colleagues(LB.ua, 2025).
Risks and ethical considerations
In recent years, cannabis has become one of the most commonly used psychoactive drugs in the United States: a large national survey estimates that 52.4 million people aged 12 and older used it last year, which is 18.7% of this age group.(Le Foll et al., n.d.). At the same time, 16.3 million people (5.8% of the same age groups) met the clinical criteria for a "cannabis use disorder". (CUD) (Le Foll et al., n.d.).
These facts can be explained by factors that influence the development of addiction. First, the earlier a person starts using cannabis, the faster their habit can progress to chronic use and eventually to CUD (Le Foll et al., n.d.). Second, although CUD incidents are more common in men across all age groups, some evidence suggests that women may develop dependence more quickly after their first use of cannabis(Le Foll et al., n.d.).
Epidemiological studies show that cannabis use significantly increases the risk of developing psychotic disorders. Thus, a meta-analysis of prospective studies showed that those who had ever tried cannabis were 2.58 times more likely to develop a psychotic disorder than those who had not.(Moore et al., 2007).

These results emphasize:
- Cannabis is a risk factor for psychosis. Even single use increases the risk several times.
- Adolescents are the most vulnerable group. The development of the brain makes them particularly sensitive to the psychotogenic effects of THC.
- Preventive measures are needed. Delaying the start of use and informing about the risks should be a priority in working with young people.
Cannabis and Ukraine - instead of a conclusion
For Ukraine to be able to effectively and safely use cannabis in the treatment of mental (and other) diseases, we need a comprehensive research program: from preclinical testing of pharmacological properties to large-scale clinical trials involving the military and civilians. At the same time, it is necessary to create clear legal rules and standards - who can receive cannabis-based medicines and for what indications, how the quality of the drugs will be controlled and how abuse will be prevented. Without a broad public education campaign, it is impossible to achieve public trust: it is important to explain to citizens that therapeutic cannabis is not a "free drug" but a carefully regulated medicine with proven efficacy and safety.
Physicians should become agents of change before prescribing therapy to a patient; they should understand the mechanisms of action of cannabinoids and be able to assess the feasibility and risks for each individual. Psychologists will help integrate cannabinoid therapy with psychotherapeutic techniques, enhancing the effect of both approaches. Scientists - pharmacologists, clinical researchers, epidemiologists - should work on continuous data collection and improvement of treatment protocols. The joint work of these specialists will result in a reliable, evidence-based algorithm that will minimize risks and maximize patient benefit.
Veterans and civilians who have experienced the trauma of war are often overlooked by traditional healthcare systems. Medical cannabis can be an important tool in reducing post-traumatic anxiety, improving sleep and overall well-being. The creation of specialized support centers where veterans and affected civilians can receive medical, psychological and social assistance with the possibility of cannabinoid therapy can give a new impetus to the recovery of both individuals and entire communities. Such a model of combining evidence-based medicine and social rehabilitation will show an example of an integrated approach that can make a real difference in the lives of those who have sacrificed their health for our safety.
Cannabinoids are not a universal remedy for all mental suffering, but they open up new opportunities to support survivors of war and other traumas as an additional tool in the hands of doctors and psychotherapists. At the same time, it is necessary to find a golden mean between strict scientific control of each stage of research and a humanistic desire to respond to the common pain and suffering of citizens, not limited to protocols and numbers. At a time when the war is accelerating the revision of our understanding of mental health and prompting us to look for atypical ways to help, it is the combination of evidence-based medicine and a humane approach that can become a real catalyst for changes in society's attitude to psychoactive substances and care for the patient's mental health.
Sources
- Di Marzo, V. (2009). The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological Research, 60(2), 77–84. https://doi.org/10.1016/j.phrs.2009.02.010
- Lu, H. C., & Mackie, K. (2021). An Introduction to the Endogenous Cannabinoid System. Biological Psychiatry, 89(6), 516-525. https://doi.org/10.1016/j.biopsych.2021.01.001
- Wilson, R. I., & Nicoll, R. A. (2002). Endocannabinoid signaling in the brain. Science, 296(5568), 678-682. https://doi.org/10.1126/science.1063545
- Maldonado, R., Valverde, O., & Berrendero, F. (2006). Involvement of the endocannabinoid system in drug addiction. Trends in Neurosciences, 29(4), 225-232. https://doi.org/10.1016/j.tins.2006.01.008
- Sidhpura, N., & Parsons, L. H. (2011). Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. https://doi.org/10.1016/j.neuropharm.2011.05.034
- Bitencourt, R. M., & Takahashi, R. N. (2018). Cannabidiol as a Therapeutic Alternative for Post-traumatic Stress Disorder: From Bench Research to Confirmation in Human Trials. Behavioural Pharmacology, 29(2 and 3‐Spec Issue), 239–251. https://doi.org/10.3389/fnins.2018.00502
- Chayasirisobhon, S. (2020). Mechanisms of action and pharmacokinetics of cannabis. The Permanente Journal, 25, 1–3. https://doi.org/10.7812/TPP/19.200
- Harbich, M. A., Michalak, S. S., & Michalak, L. M. (2024). Understanding Medical Cannabis: A simple guide to CBD and THC. Acta Poloniae Pharmaceutica, 81(3), 595-601. ⦁ https://www.ptfarm.pl/en/wydawnictwa/czasopisma/acta-poloniae-pharmaceutica/110/-/30574)[3]
- Wright, M., Di Ciano, P., & Brands, B. (2020). Use of cannabidiol for the treatment of anxiety: A short synthesis of pre-clinical and clinical evidence. Canadian Journal of Addictions, 11(3), 34–42. https://sgcm-sscm.ch/wp-content/uploads/2024/06/wright_ccr-2020_cbd-anxiety.pdf
- Pertwee, R. G. (2008). Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addiction Biology, 13(2), 147–159. https://doi.org/10.1111/j.1369-1600.2008.00108.x
- Bisogno, T., Hanus, L., De Petrocellis, L., Tchilibon, S., Ponde, D. E., Brandi, I., ... & Di Marzo, V. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology, 134(4), 845–852 https://doi.org/10.1038/sj.bjp.0704327
- De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, M., Bisogno, T., Petrosino, S., ... & Di Marzo, V. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163(7), 1479–1494. https://doi.org/10.1111/j.1476-5381.2010.01166.x
- Leweke, F. M., Piomelli, D., Pahlisch, F., Muhl, D., Gerth, C. W., Hoyer, C., ... & Koethe, D. (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational Psychiatry, 2, e94. https://doi.org/10.1038/tp.2012.15
- Elmes, M. W., Kaczocha, M., Berger, W. T., Leung, K., Ralph, B. P., Wang, L., ... & Deutsch, D. G. (2015). Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Journal of Biological Chemistry, 290(14), 8711–8721. https://doi.org/10.1074/jbc.M114.618447
- Chhatwal, J. P., Davis, M., Maguschak, K. A., & Ressler, K. J. (2005). Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology, 30(3), 516–524. https://doi.org/10.1038/sj.npp.1300624
- Pamplona, F. A., Prediger, R. D., Pandolfo, P., & Takahashi, R. N. (2006). The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology, 188, 641–649. http://dx.doi.org/10.1007/s00213-006-0514-0
- Bitencourt, R. M., & Takahashi, R. N. (2008). Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol. European Neuropsychopharmacology, 18(12), 849–859. https://doi.org/10.1016/j.euroneuro.2008.07.001
- Bitencourt, R. M., & Takahashi, R. N. (2018). Cannabidiol as a Therapeutic Alternative for Post‐traumatic Stress Disorder: From Bench Research to Confirmation in Human Trials. Frontiers in Neuroscience, 12, 502. https://doi.org/10.3389/fnins.2018.00502
- Russo, E., Burnett, A., Hall, B., & Parker, K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochemical Research, 30(8), 1037–1043. https://doi.org/10.1007/s11064-005-6978-1
- Campos, A. C., & Guimarães, F. S. (2008). Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology, 199(2), 223–230. https://doi.org/10.1007/s00213-008-1168-x
- Gomes, F. V., Resstel, L. B., & Guimarães, F. S. (2011). The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology, 213(2-3), 465–473. https://doi.org/10.1007/s00213-010-2036-z
- Campos, A. C., & Guimarães, F. S. (2009). Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Progress in neuro-psychopharmacology & biological psychiatry, 33(8), 1517–1521. https://doi.org/10.1016/j.pnpbp.2009.08.017
- Campos, A. C., Moreira, F. A., Gomes, F. V., Del Bel, E. A., & Guimarães, F. S. (2012b). Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1607), 3364–3378. https://doi.org/10.1098/rstb.2011.0389
- Resstel, L. B., Tavares, R. F., Lisboa, S. F., Joca, S. R., Corrêa, F. M., & Guimarães, F. S. (2009). 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. British Journal of Pharmacology, 156(1), 181–188. https://doi.org/10.1111/j.1476-5381.2008.00046.x
- Stella, B., Baratta, F., Della Pepa, C., Arpicco, S., Gastaldi, D., & Dosio, F. (2021). Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs, 81(13), 1513–1557. https://doi.org/10.1007/s40265-021-01579-x
- Kaczorová D, Peč J, Béres T, Štefelová N, Ćavar Zeljković S, Trojan V, Janatová AK, Klouček P and Tarkowski P (2023), Phytocannabinoid-rich galenic preparations for topical administration: extraction and stability testing. Front. Pharmacol. 14:1230728. doi: 10.3389/fphar.2023.1230728
- Blessing, E. M., Steenkamp, M. M., Manzanares, J., & Marmar, C. R. (2015). Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics, 12(4), 825–836. https://doi.org/10.1007/s13311-015-0387-1
- Lee, J. L. C., Bertoglio, L. J., Guimarães, F. S., & Stevenson, C. W. (2017). Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. British Journal of Pharmacology, 174(19), 3242–3256. https://doi.org/10.1111/bph.13724
- Papagianni, E. P., & Stevenson, C. W. (2019). Cannabinoid Regulation of Fear and Anxiety: an Update. Current Psychiatry Reports, 21, 38. https://doi.org/10.1007/s11920-019-1026-z
- Bertoglio, Leandro & Carobrez, Antonio. (2016). Animal Tests for Anxiety. Animal models as tools in ethical biomedical research. 271-284. 10.1007/978-3-319-11578-8_18.
- Berger, W., Mendlowicz, M. V., Marques-Portella, C., Kinrys, G., Fontenelle, L. F., Marmar, C. R., & Figueira, I. (2009). Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Progress in neuro-psychopharmacology & biological psychiatry, 33(2), 169–180. https://doi.org/10.1016/j.pnpbp.2008.12.004
- Shin, L. M., Rauch, S. L., & Pitman, R. K. (2014). Posttraumatic stress disorder: Neuroimaging and neurocircuitry. Neuropsychopharmacology, 39(1), 1–23. https://doi.org/10.1017/S1092852900007306
- Bernardy, N. C., & Friedman, M. J. (2017). Pharmacological management of posttraumatic stress disorder. Current Opinion in Psychology, 14, 116–121. https://doi.org/10.1016/j.copsyc.2017.01.003
- Marsicano, G., Wotjak, C. T., Azad, S. C., Bisogno, T., Rammes, G., Cascio, M. G., ... & Lutz, B. (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418(6897), 530–534. https://doi.org/10.1038/nature00839
- Quirk, G. J., & Mueller, D. (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology, 33(1), 56–72. https://doi.org/10.1038/sj.npp.1301555
- Singewald, N., Schmuckermair, C., Whittle, N., Holmes, A., & Ressler, K. J. (2015). Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacology & Therapeutics, 149, 150–190. https://doi.org/10.1016/j.pharmthera.2014.12.004
- Levin, R., Almeida, V., Peres, F. F., Calzavara, M. B., da Silva, N. D., Suiama, M. A., Niigaki, S. T., Zuardi, A. W., Hallak, J. E., Crippa, J. A., & Abílio, V. C. (2012). Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Current pharmaceutical design, 18(32), 4960–4965. https://doi.org/10.2174/138161212802884735
- Stern, C. A. J., Gazarini, L., Takahashi, R. N., Guimarães, F. S., & Bertoglio, L. J. (2012). On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology, 37(9), 2132–2142. https://doi.org/10.1038/npp.2012.63
- Bitencourt, R. M., Pamplona, F. A., & Takahashi, R. N. (2008). Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol. European Neuropsychopharmacology, 18(12), 849–859. https://doi.org/10.1016/j.euroneuro.2008.07.001
- Do Monte, F. H. M., Souza, R. R., Bitencourt, R. M., Kroon, J. A., & Takahashi, R. N. (2013). Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behavioural Brain Research, 250, 23–27. https://doi.org/10.1016/j.bbr.2013.04.045
- Fusar-Poli, P., Crippa, J. A., Bhattacharyya, S., Borgwardt, S. J., Allen, P., Martin-Santos, R., Seal, M., Surguladze, S. A., O'Carrol, C., Atakan, Z., Zuardi, A. W., & McGuire, P. K. (2009). Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Archives of general psychiatry, 66(1), 95–105. https://doi.org/10.1001/archgenpsychiatry.2008.519
- Passie, T., Emrich, H. M., Karst, M., Brandt, S. D., & Halpern, J. H. (2012). Mitigation of post-traumatic stress symptoms by Cannabis resin: A review of the clinical and neurobiological evidence. Drug Testing and Analysis, 4(7–8), 649–659. https://doi.org/10.1002/dta.1377
- Jiang, W., Zhang, Y., Xiao, L., Van Cleemput, J., Ji, S. P., Bai, G., & Zhang, X. (2005). Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. The Journal of clinical investigation, 115(11), 3104–3116. https://doi.org/10.1172/JCI25509
- Monti J. M. (1977). Hypnoticlike effects of cannabidiol in the rat. Psychopharmacology, 55(3), 263–265. https://doi.org/10.1007/BF00497858
- Hsiao, Y. T., Yi, P. L., Li, C. L., & Chang, F. C. (2012). Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology, 62(1), 373–384. https://doi.org/10.1016/j.neuropharm.2011.08.013
- Chagas, M. H., Eckeli, A. L., Zuardi, A. W., Pena-Pereira, M. A., Sobreira-Neto, M. A., Sobreira, E. T., ... & Crippa, J. A. (2014). Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: A case series. Journal of Clinical Pharmacy and Therapeutics, 39(5), 564–566. https://doi.org/10.1111/jcpt.12177
- Chesney, E., Oliver, D., Green, A., Sovi, S., Wilson, J., Englund, A., & Freeman, T. P. (2020). Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology, 45(11), 1799–1806. https://doi.org/10.1038/s41386-020-0661-8
- Gaston, T. E., & Friedman, D. (2017). Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy & behavior : E&B, 70(Pt B), 313–318. https://doi.org/10.1016/j.yebeh.2016.11.016
- Geffrey, A. L., Pollack, S. F., Bruno, P. L., & Thiele, E. A. (2015). Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia, 56(8), 1230–1236. https://doi.org/10.1111/epi.13063
- Morrison, G., Crockett, J., Blakey, G., & Sommerville, K. (2019). A phase 1, open-label, pharmacokinetic trial to investigate possible drug–drug interactions between cannabidiol and clobazam, stiripentol, or valproate in healthy subjects. Clinical Pharmacology in Drug Development, 8(8), 1009–1031. https://doi.org/10.1002/cpdd.691
- Masataka, N. (2019). Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Frontiers in Psychology, 10, 2466. https://doi.org/10.3389/fpsyg.2019.02466
- Arndt, D. L., & de Wit, H. (2017). Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis and Cannabinoid Research, 2(1), 105–113. https://doi.org/10.1089/can.2017.0016
- Ibeas Bih, C., Chen, T., Nunn, A. V., Bazelot, M., Dallas, M., & Whalley, B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics, 12(4), 699–730. https://doi.org/10.1007/s13311-015-0377-3
- Cascio, M. G., Anavi-Goffer, S., Fletcher, P. J., Mechoulam, R., Pertwee, R. G., & Parker, L. A. (2012). Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. British journal of pharmacology, 165(8), 2620–2634. https://doi.org/10.1111/j.1476-5381.2011.01621.x
- Brandon Utter, C Alan Anderson, Christopher M Filley, James P Kelly, Catharine Johnston-Brooks, David B Arciniegas, Cannabis Use in a Cohort of Healthcare-Seeking United States Military Veterans With Persisting Symptoms After Mild Traumatic Brain Injury: Preliminary Observations, Military Medicine, Volume 188, Issue 7-8, July/August 2023, Pages e2158–e2164, https://doi.org/10.1093/milmed/usac011
- Betthauser, K., Pilz, J., & Vollmer, L. E. (2015). Use and effects of cannabinoids in military veterans with posttraumatic stress disorder. American Journal of Health-System Pharmacy, 72(15), 1279-1284. https://doi.org/10.2146/ajhp140523
- Jackson, K. M., & Borsari, B. (2018). Medicinal versus Recreational Cannabis Use among Returning Veterans. Translational issues in psychological science, 4(1), 6–20. https://doi.org/10.1037/tps0000133
- Bonn-Miller, M. O., Sisley, S., Riggs, P., Yazar-Klosinski, B., Wang, J. B., Loflin, M. J., Shechet, B., Hennigan, C., Matthews, R., DeWitt, A., & Doblin, R. (2021). The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS One, 16(3), e0246990. https://doi.org/10.1371/journal.pone.0246990
- VFW. (2021, September 21). Federal study finds cannabis beneficial for PTSD treatment. VFW. https://www.vfw.org/media-and-events/latest-releases/archives/2021/9/federal-study-finds-cannabis-beneficial-for-ptsd-treatment
- Politico. (2021, November 8). VA rejects cannabis research as veterans plead for medical pot. Politico. https://www.politico.com/news/2021/11/08/va-reject-medical-marijuana-veterans-519757
- Stars and Stripes. (2024, November 22). FDA authorizes clinical trials to study cannabis use for veterans with PTSD. Stars and Stripes. https://www.stripes.com/veterans/2024-11-22/veterans-marijuana-ptsd-trials-fda-15935021.html
- The Psychiatrist. (2024, December 9). FDA approves cannabis study for veterans with PTSD. The Psychiatrist. https://www.psychiatrist.com/news/fda-approves-cannabis-study-for-veterans-with-ptsd/
- Yakirevich Amir, N., Treves, N., Davidson, E., Bonne, O., & Matok, I. (2023). Medical cannabis use among patients with Post-Traumatic Stress Disorder (PTSD): A nationwide database study. European Psychiatry, 66(Suppl 1), S111–S112. https://doi.org/10.1192/j.eurpsy.2023.306
- Globes. (2025, January 9). Is medical cannabis effective in treating PTSD? Globes. https://www.globes.co.il/news/article.aspx?did=1001499062
- Clinical Trial. (2022, February 16). Clinical trial highlights positive effects of medical cannabis on PTSD. MedPath. https://trial.medpath.com/news/ab2258ab47d5f3d9/clinical-trial-highlights-positive-effects-of-medical-cannabis-on-ptsd
- NoCamels. (2023, February 16). Smoke free: Israeli veterans get cannabis inhaler. NoCamels. https://nocamels.com/2023/02/smoke-free-israeli-veterans-get-cannabis-inhaler/
- Syqe Medical. (2025, February 20). Syqe Medical announces agreement with Israeli Ministry of Defence to provide the SyqeAir technology to veterans. Syqe Medical. https://syqe.com/syqe-medical-announces-agreement-with-israeli-ministry-of-defence-to-provide-the-syqeair-technology-to-veterans/
- Veterans Affairs Canada. (2025, April 29). Cannabis for medical purposes. Veterans Affairs Canada. https://www.veterans.gc.ca/en/about-vac/reports-policies-and-legislation/departmental-reports/cannabis-medical-purposes
- Walsh, Z., Gonzalez, R., Crosby, K., Thiessen, M. S., Carroll, C., & Bonn-Miller, M. O. (2017). Medical cannabis and mental health: A guided systematic review. Clinical Psychology Review, 51, 15-29. https://doi.org/10.1016/j.cpr.2016.10.002
- Valikhanova, G., Kato, Y., Fitzcharles, M. A., Ware, M., Da Costa, D., Lowensteyn, I., Cheung, H. S., & Grover, S. (2023). Medical Cannabis Use Among Canadian Veterans and Non-Veterans: A National Survey. Integrative medicine reports, 2(1), 120–128. https://doi.org/10.1089/imr.2023.0022
- Mental Health Commission of Canada. (2023, February 22). Veterans and their families want better research and clinical support on cannabis as a treatment option for their mental health. Mental Health Commission of Canada. https://mentalhealthcommission.ca/news-releases/56112-veterans-and-their-families-want-better-research-and-clinical-support-on-cannabis-as-a-treatment-option-for-their-mental-health/
- Business Insider. (2023, December 22). Ukraine is legalizing medicinal marijuana to treat stress and cancer caused by war. Business Insider. https://www.businessinsider.com/ukraine-legalize-marijuana-cannabis-medicinal-ptsd-cancer-war-2023-12
- Le Monde. (2024, September 14). Ukraine's legalization of medical cannabis is a source of hope for veterans. Le Monde. https://www.lemonde.fr/en/international/article/2024/09/14/ukraine-s-legalization-of-medical-cannabis-is-a-source-of-hope-for-veterans_6726042_4.html
- Cannabis Health News. (2024, October 9). Ukraine excludes PTSD from list of conditions for medical cannabis. Cannabis Health News. https://cannabishealthnews.co.uk/2024/10/09/ukraine-excludes-ptsd-from-list-of-approved-conditions-for-medical-cannabis/ Soft Secrets. (2025, May 1).
- Cannabis in times of war. Soft Secrets. https://softsecrets.com/en-US/article/cannabis-times-war
- BBC Ukraine (2023, December 21). Cannabis legalized in Ukraine. How to get the medicine. BBC News Ukraine.https://www.bbc.com/ukrainian/articles/cw4kwv45z51o
- Gvara Media (2025, January 8). In 2025, the first cannabis medicines will appear in Ukraine. Guara Media. https://gwaramedia.com/u-2025-roczi-v-ukrayini-zyavlyatsya-pershi-liki-z-kanabisu/
- Hromadske Radio (2025, January 9). How to get medicines based on medical cannabis. Hromadske Radio.https://hromadske.radio/news/2025/01/09/yak-otrymaty-liky-na-osnovi-medkanabisu
- Cabinet of Ministers of Ukraine (2023, December 21). The Parliament adopted the draft law No. 7457 on the use of cannabis-based medicines in medicine. Government portal. https://www.kmu.gov.ua/news/parlament-ukhvalyv-zakonoproekt-7457-pro-vykorystannia-v-medytsyni-likiv-na-osnovi-kanabisu
- CMU (2025, January 1). The government has approved license conditions regulating the circulation of medical cannabis at all stages. Government portal.https://www.kmu.gov.ua/news/uriad-zatverdyv-litsenziini-umovy-shcho-vrehulovuiut-obih-medychnoho-kanabisu-na-vsikh-etapakh
- Kovalenko, A., Pakhomov, I., Horoshko, O., Kalinichenko, V., & Bezditko, P. (2020). Perspectives on formation of medical cannabis market in Ukraine based on holistic approach. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC7819340/
- LB.ua (2025, January 8). First cannabis medicines registered in Ukraine. LB.ua. https://lb.ua/health/2025/01/08/654295_ukraini_zareiestrovani_pershi_liki_z.html
- Life.pravda. (2025, February 11). Medical cannabis in Ukraine: what has already changed for patients and businesses. Ukrainska Pravda. Life. https://life.pravda.com.ua/society/medichniy-kanabis-v-ukrajini-shcho-vzhe-zminilosya-dlya-paciyentiv-ta-biznesu-306383/
- MedPlatforma. (2024, October 1). The Ministry of Health has approved the list of diseases and conditions for which medical cannabis should be prescribed. MedPlatforma. https://medplatforma.com.ua/news/83598-moz-zatverdilo-perelik-khvorob-i-staniv-za-yakikh-priznachati-medichniy-kanabis
- Ministry of Health. (2024, August 16). Medical cannabis. Ministry of Health of Ukraine. https://moz.gov.ua/uk/medichnij-kanabis
- Ministry of Health (2024, September 27). The Ministry of Health approved the list of diseases and conditions for which medical cannabis will be prescribed. Ministry of Health of Ukraine.https://moz.gov.ua/uk/moz-zatverdilo-perelik-hvorob-i-staniv-za-yakih-priznachatimetsya-medichnij-kanabis
- Ministry of Health (2024, November 5). Medical cannabis: the government has set deadlines for applications for quotas in 2025. Ministry of Health of Ukraine. https://moz.gov.ua/uk/medichnij-kanabis-uryad-viznachiv-termini-podannya-zayav-na-otrimannya-kvot-u-2025-roci
- Rada. (2025, August 1). Mikhail Radutsky: Only medical cannabis is legalized in Ukraine. Verkhovna Rada of Ukraine. https://www.rada.gov.ua/news/news_kom/245144.html
- Studfreedom. (2023, December 1). Medical cannabis - to be or not to be? Student Freedom.https://studfreedom.org/ua/medychnomu-kanabisu-buty-chy-ne-buty/
- Suspilne (2024, August 16). Legalization of medical cannabis: what will change for those who need it. Public Broadcasting.https://suspilne.media/uzhhorod/815131-legalizacia-medicnogo-kanabisu-so-zminitsa-dla-tih-hto-jogo-potrebuvatime/
- Suspilne (2025, June 3). For the first time in Ukraine, a permit for the import of medical cannabis was issued. Public broadcasting. https://suspilne.media/1033787-v-ukraini-vperse-vidali-dozvil-na-vvezenna-medicnogo-kanabisu/
- TSN (2024, September 27). For what diseases will Ukrainians be prescribed medical cannabis. TSN.https://tsn.ua/zdorovya/za-yakih-hvorob-ukrayincyam-priznachatimut-medichniy-kanabis-moz-2668695.html
- The Village. (2025, April 5). The Ministry of Health has approved the list of diseases and conditions for which medical cannabis will be prescribed. The Village Ukraine.https://www.village.com.ua/village/life/edu-news/355295-moz-zatverdilo-perelik-hvorob-i-staniv-za-yakih-priznachatimut-medichniy-kanabis
- Ukr.radio. (2024, August 19). The first medicines will be available by the end of the year - Nickel on medical cannabis. Ukrainian Radio. https://ukr.radio/news.html?newsID=105081
- Ukrinform (2024, August 16). The law on the legalization of medical cannabis came into force in Ukraine. Ukrinform.https://www.ukrinform.ua/rubric-society/3895695-v-ukraini-pocav-diati-zakon-pro-legalizaciu-medicnogo-kanabisu.html
- UNIAN (2023, July 7). The Ministry of Health supports the legalization of medical cannabis in Ukraine. UNIAN. https://www.unian.ua/health/legalizaciya-medichnogo-kanabisu-moz-pidtrimav-ideyu-12320427.html
- Le Foll, B., et al. (2024). Cannabis use disorder: from neurobiology to treatment. The Journal of Clinical Investigation, 134(20): e172887. https://doi.org/10.1172/JCI172887.
- Moore, T. H. M., Zammit, S., Lingford-Hughes, A., Barnes, T. R. E., Jones, P. B., Burke, M., & Lewis, G. (2007). Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. The Lancet, 370(9584), 319–328. https://doi.org/10.1016/S0140-6736(07)60959-6
- Lubman, D. I., Cheetham, A., & Yücel, M. (2015). Cannabis and adolescent brain development. Pharmacology & therapeutics, 148, 1–16. https://doi.org/10.1016/j.pharmthera.2014.11.009
- McDonald, A. J., Kurdyak, P., Rehm, J., Roerecke, M., & Bondy, S. J. (2024). Age-dependent association of cannabis use with risk of psychotic disorder. Psychological medicine, 54(11), 2926–2936. https://doi.org/10.1017/S0033291724000990